Anti-obesity Medication (NovOB)
Pipeline
NovOB
Program Target
Obesity
Patient Numbers
1.9 Billion
Development Stage
Clinical Phase II
NovMetaPharma develops NovOB, an anti-obesity medication of first-in-class new substance.
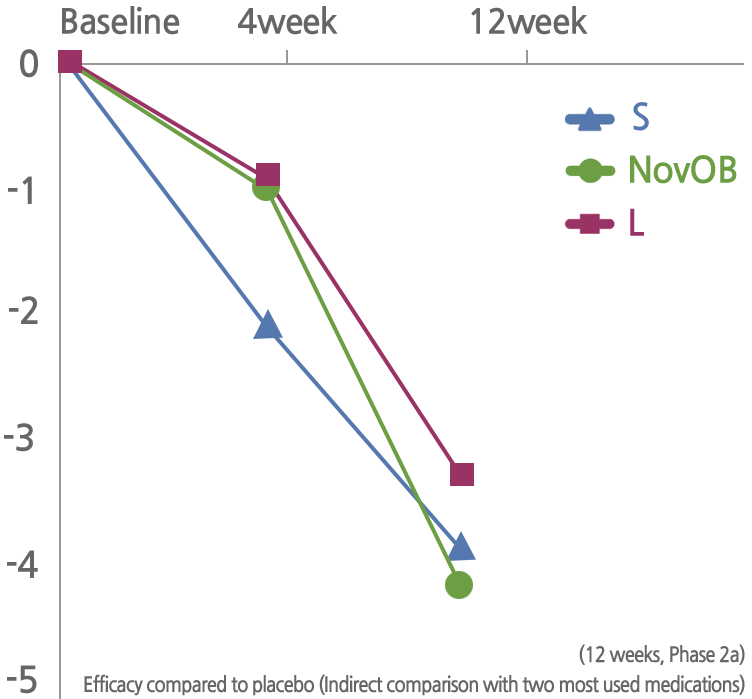
NovOB’s excellent weight loss efficacy and long-term safety have been proven through several clinical and non-clinical trials.
Existing commercial anti-obesity medications have side effects and risks and long-term use is almost impossible as most of them are psychotropic. Also, in the case of the product that takes up the highest market share among anti-obesity medication currently available on the market, the inconvenience of self-injection is pointed out as a major disadvantage.
On the other hand, NovOB is guaranteed to be safe as it is a peptide substance existing in the body and this has been proven through several clinical and non-clinical trials. NovOB reduces weight with just one oral administration a day. As a once-a-day oral medication, NovOB is very convenient to take and patient convenience has been greatly improved by dramatically shortening the dosing interval.
01
Weight loss effect
Significant weight loss effect has been confirmed through the US FDA clinical phase 2 (including a and b).
02
Safety
Safety such as chronic toxicity and reproductive toxicity has been verified through several G140 global clinical and non-clinical trials.
03
Administration convenience
Oral (not injection)

 Outline
Outline
 Diabetes Medication
Diabetes Medication 
 Anti-obesity Medication
Anti-obesity Medication 
 Kidney Disease Medication
Kidney Disease Medication 
 Fibrosis Medication
Fibrosis Medication 
 Retroperitoneal Fibrosis Medication
Retroperitoneal Fibrosis Medication